Xanomeline is a very unique antipsychotic given the fact that it does not antagonize D2 in any appreciable manner, and does not act directly on any dopamine receptors. Instead, it is a muscarinic receptor agonist with high affinity for M1 and M4 receptors. Theoretically, it has action in every single muscarinic receptor, but it’s considered functionally selective for M1 and M4 at therapeutic doses. Unlike M2/3/5, the M1 and M4 receptors are primarily located in cortical and limbic regions of the brain, and these regions of the brain include the dopaminergic pathways associated with schizophrenia.1
According to radiotracer studies, M1 receptors are diminished in the dorsolateral prefrontal cortex of schizophrenic patients, but preserved in the rest of the brain1. Cholinergic markers also appear to be normal in schizophrenic patients.
In the prefrontal cortex, M1 agonism/positive allosteric modulation (PAM) leads to acts as a stimulator of gabaergic interneurons that inhibit glutamate signalling in the prefrontal cortex, this inhibition leads to lower excitation of dopaminergic neurons, and diminished glutamatergic signalling to the midbrain1, 2 (Figure 1.)
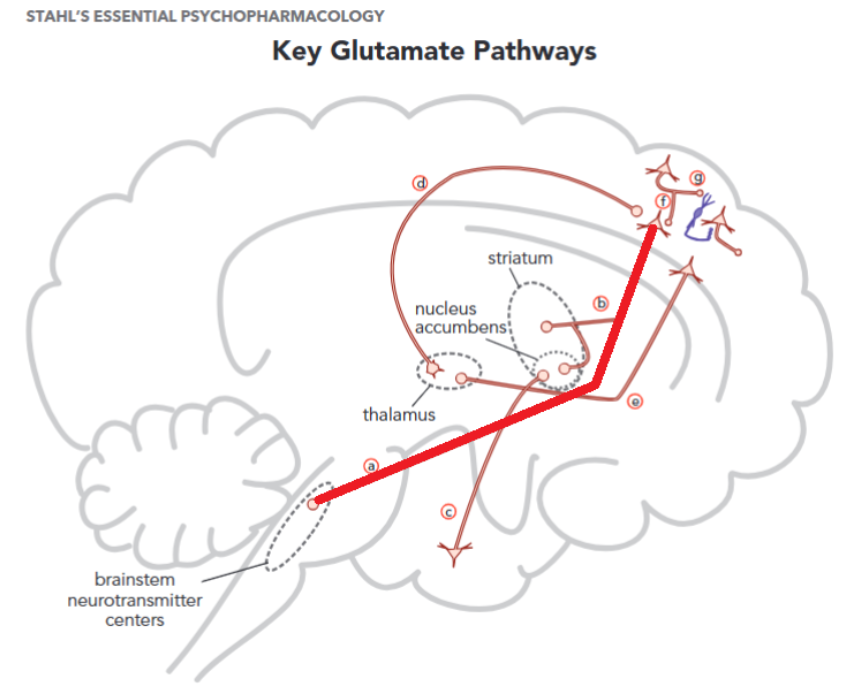
M4 acts as an inhibitory autoreceptor for Ach neurons in the laterodorsal tegmental nucleus, these neurons stimulate other dopaminergic neurons.. By diminishing the activity in these stimulating neurons, there is less presynaptic dopamine release thereby diminishing positive symptoms. It is important to note that most antipsychotics act postsynaptically by blocking the D2 receptor. For example, in the nucleus accumbens and ventral striatum the dopamine release gets inhibited through M4 agonism.
A couple of papers that talk about dopamine regulation by M4 mention that it also leads to higher dopamine release in the hippocampus and “cortex”4 (No specification on which part of the cortex, or if it was the prefrontal cortex or just general cortex), they cite one paper in particular5, but I could not actually see any “dopamine release enhancement”. It is known that xanomeline enhances dopamine release in the medial prefrontal cortex4, which, to the best of my knowledge, is through an unknown mechanism. INTERESTING STUFF
Other neurobiology facts about M4 is that it is highly expressed throughout the substantia nigra, which is involved in dopaminergic signalling throughout the brain.forgot citation will fix There is also a diminished number of M4 receptors in the caudate-putamen and hypothalamus in schizophrenic patients, and genetic variations in the M4 receptor are linked with schizophrenia
Other muscarinic agonists are being tested, such as Emraclidine (an M4 PAM) and NBI-1117568 (a selective M4 agonist). Emraclidine failed to meet the primary endpoints in phase II clinical trials, suggesting a significant role for M1 receptor modulation and/or agonism. Also, NBI-1117568 had positive results in its phase II trials for only one of four doses studied, with the 20 mg OD dose having statistically significant positive results, but non-statistically significant results for the 40 mg OD, 30 mg BID and 60 mg OD doses.6, 7 This points to M1 having an important role for the antipsychotic effects of Xanomeline, or perhaps even M2/3/5 activity being important. Maybe M4 agonism is required on top of M4 positive allosteric modulation. Nobody knows for sure!
History/Big Pharma stuff
This drug was originally developed in 1994 by Eli Lilly for the treatment of Alzheimer's disease. It made sense then because M1 receptors are generally preserved throughout the course of the disease and, unlike acetylcholinesterase inhibitors, it does not require intact presynaptic nerve terminals for its therapeutic effects. It was hypothesized that restoring function for M1 could restore some cognitive function. Xanomeline showed some efficacy, particularly in psychotic syndromes, but did not pass clinical trials due to significant safety issues in the elderly. Agonism of M1 receptors outside of the brain led to gastrointestinal adverse events, mostly nausea, salivation and vomiting.8, 9, 10
Karuna Pharmaceuticals licensed this drug from Eli Lilly and started doing clinical trials for KarXT, a combination of Xanomeline and Trospium Chloride, a peripheral, non-specific muscarinic antagonist. After being acquired by Bristol Myers Squibb, the FDA approved this drug: the first non-D2 antipsychotic.10
Clozapine and the muscarinic system.
Clozapine is a fascinating compound because we have no idea how it workscitation needed, it has yet to be replicated and it’s difficult to explain its (low, but existent) efficacy in treatment resistant schizophrenia11. I decided to place Clozapine, an atypical, alongside Xanomeline in this series because of something that peaks my interest.
Clozapine is known to be strongly anticholinergic, but it does not decrease and appears to even increase the cognitive capabilities of schizophrenic patients.9 Now, this could be due cognitive gains associated with successful antipsychotic treatments in spite of the anticholinergic effects, but it could also be a phenomenon associated with the metabolites of Clozapine, namely N-desmethylclozapine/Norclozapine. Plasma levels of Norclozapine are comparable if not higher than clozapine itself, indicating a potential role. This metabolite has a rather high affinity and intrinsic activity (~50%) at the M1 receptor, it is a partial agonist! And because the concentration of Norclozapine is as high as clozapine itself, there is a pretty good possibility that it actually makes up for the anticholinergic effects of clozapine.9
What is the possibility that clozapine, a strongly anticholinergic drug, actually behaves as an agonist at the M1 receptor? I immediately started thinking about the lack of efficacy for M4 receptor PAMs implying a need for M1 agonism/PAM. Right now all of these things are unknown.
There is a lot of research to be done in the realm of cholinergic drugs and I hope that the approval of Xanomeline leads to a lot more research in this area.
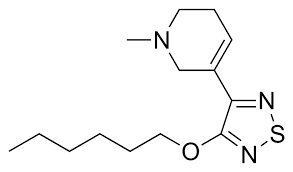
Epilogue:
It took me a long time to write all this about schizophrenia and I feel like I have barely covered the tip of the iceberg, there are so many things I want to talk about such as the mechanisms behind the safety issues in APs, the neurology behind salience, etc. I even completely ignored the antagonism of 5-HT2a as a mechanism for antipsychotic action because I wanted to be done with it. Right now I will probably take a big break from schizophrenia literature, but there is a pretty good chance I will go back to this later in the future.
-
References
- Ali Azargoonjahromi, “Current Findings and Potential Mechanisms of KarXT (Xanomeline–Trospium) in Schizophrenia Treatment”, 2024. DOI: 10.1007/s40261-024-01377-9
- Jonathan M. Meyer, “How antipsychotics work in schizophrenia: a primer on mechanisms”, 2024. DOI: 10.1017/S1092852924002244
- S. Stahl, “Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications”, 5th ed, 2021.
- S. Jones, P. Harvey, “Cross-diagnostic determinants of cognitive functioning: the muscarinic cholinergic receptor as a model system”, 2023. DOI: 10.1038/s41398-023-02400-x
- M. Bubser, T. Bridges, D. Dencker, et al. “Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents”, 2014. DOI: 10.1021/cn500128b
- Leah Kuntz, “Emraclidine for Schizophrenia Fails to Meet Primary Endpoints in Phase 2 EMPOWER Trials”, 2024. Obtained from: https://www.psychiatrictimes.com/view/emraclidine-for-schizophrenia-fails-to-meet-primary-endpoints-in-phase-2-empower-trials
- Neurocrine Biosciences, “Neurocrine Biosciences Reports Positive Phase 2 Data for NBI-1117568 in Schizophrenia”, 2018. Obtained from: https://neurocrine.gcs-web.com/news-releases/news-release-details/neurocrine-biosciences-reports-positive-phase-2-data-nbi-1117568
- F. Bymaster, C. Whitesitt, H. Shannon, et al, “Xanomeline: A selective muscarinic agonist for the treatment of Alzheimer's disease”, 1997. DOI: 10.1002/(sici)1098-2299(199702)40:2<158::aid-ddr6>3.0.co;2-k
- Kidambi, O. Elsayed, R. El-Mallakh, “Xanomeline-Trospium and Muscarinic Involvement in Schizophrenia”, 2023. DOI: 10.2147/NDT.S406371
- Jeffrey A. Lieberman, “The inside story of Cobenfy”, 2024. https://www.healio.com/news/psychiatry/20241003/the-inside-story-of-cobenfy
- D. Siskind, V. Siskind, S. Kisely, “Clozapine Response Rates among People with Treatment-Resistant Schizophrenia: Data from a Systematic Review and Meta-Analysis”, 2017. DOI: 10.1177/0706743717718167