Orexins (also known as hypocretins) are a group of peptides that were independently discovered by two different groups in the late 90s1. The word orexin is derived from Greek órexis “appetite” (the same root as anorexia, i.e. without appetite). Orexins were named this way because they have been observed to be heavily involved in appetite regulation in animal models. On the other hand the name “hypocretin” is not as exciting, from hypo- (from hypothalamus) and -cretin (after secretin)1. Basically, orexins look a bit like secretin, another peptide, and they are made in the hypothalamus, hence hypocretin. These peptides are produced by highly specialized neurons, orexin neurons, which innervate many areas of the brain, including stimulating, wake-promoting areas (more on that later)1. Interestingly enough, the orexin system is present in a wide variety of species, from humans, dogs and cats to snails, geckos and fish2. With humans having around 50-80 thousand orexin neurons, while zebrafish have only around 80 orexin neurons2 (I thought this was neat).
Orexins, alongside their respective receptors, known together as the orexin system, are also involved in the sleep-wake cycle, metabolism, appetite and thermal regulation, and are the therapeutic target for several pathologies, namely insomnia and narcolepsy1,3. The discovery of orexin’s involvement in narcolepsy was due to a genetic study; Ling Lin et al2 carried out a breeding program for narcoleptic dogs and produced dogs with familial narcolepsy and cataplexy, in which strong emotions make the patient’s muscles weak. That is to say, the puppies would start playing, get excited, drop down, then fall asleep (look it up on youtube). Dogs suffering from this condition had a genetic mutation in the orexin 2 receptor (OXR2). This discovery was then confirmed by genetically modifying rats in order to remove the genes that codify for OXR2, this is known as a knock-out model, and as predicted, they too showed narcolepsy and cataplexy2 (I personally would have loved to play with narcoleptic dogs and mice). If narcolepsy were a dysfunction of orexin receptors in humans, then making a pharmacological therapy would prove to be quite difficult (no receptors to agonize = no way of targeting OXR2). Fortunately, human narcolepsy and cataplexy (also known as narcolepsy type 1) is primarily due to a lack/loss of orexin neurons and not the loss of OXR24.
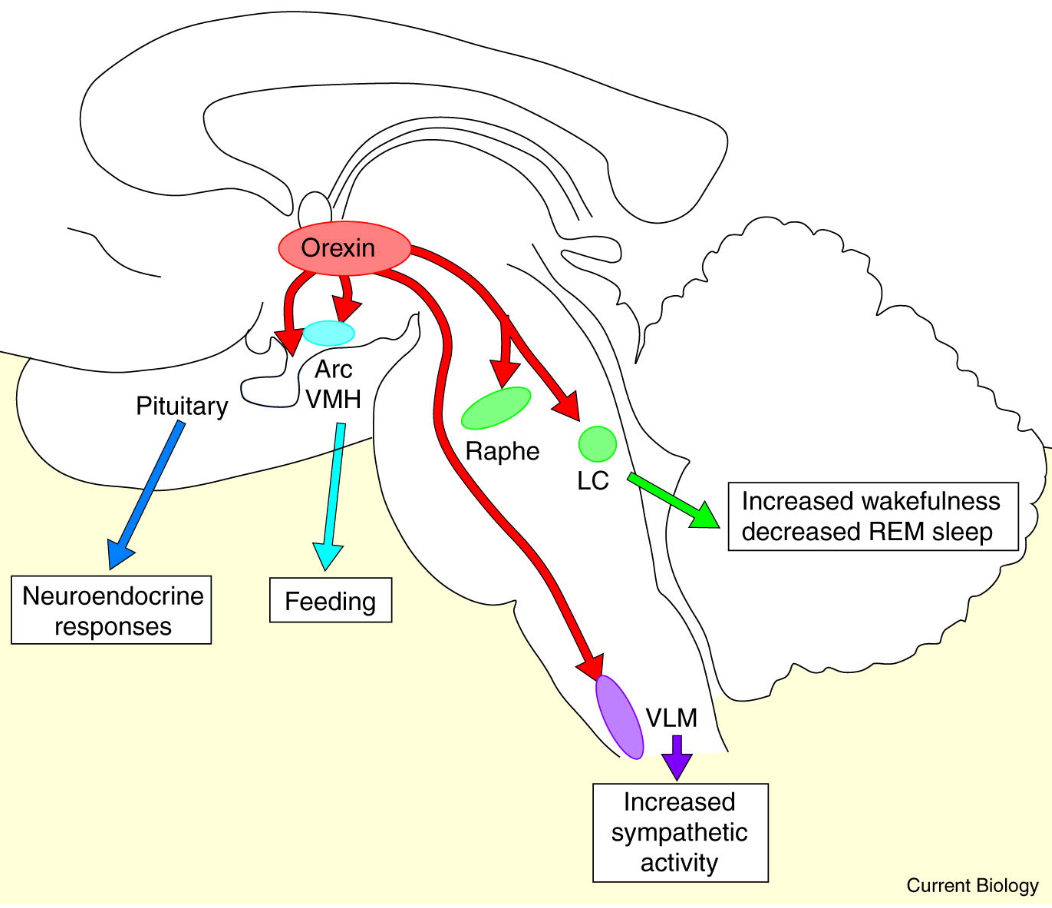
So, we have orexin A and B, which activate the orexin receptors 1 and 2, with OXR2 being heavily involved in sleep. A lack of orexin or orexin receptor dysfunction leads to narcolepsy, and dysregulation of orexin neurons is hypothesized to be implicated in the pathogenesis of insomnia.
As of 2024, there are three approved orexin antagonists – suvorexant, daridorexant and lemborexant – these antagonists will be the main focus of the article and their mechanism of action can also be used to explain the mechanism for orexin agonists.
Dual orexin antagonists: Suvorexant (Belsomra) and the -orexants.
To explain the role of orexins in insomnia and narcolepsy, I will first explain the so-called flip-flop switch model of sleep. This model postulates that there is a mutual inhibition between the brain’s sleep promoting nuclei, mainly the ventrolateral preoptic nucleus (VLPO) and excitatory, monoaminergic nuclei which include the locus coeruleus (responsible for the synthesis of noradrenaline), the raphe nucleus (responsible for serotonin signaling in the brain) and the tuberomammillary nucleus (the sole source of histamine in the brain)5. In other words, excitatory and inhibitory parts of the brain inhibit each other. As soon as one gets strong enough, the system flips over, changing from awake to sleep. The orexin system would serve as a stabilizing force in the model, reinforcing the monoaminergic nuclei to “keep the brain awake”. Then, orexin neurons are inhibited by the sleep-promoting VLPO, which allows for a stronger transition between states5.
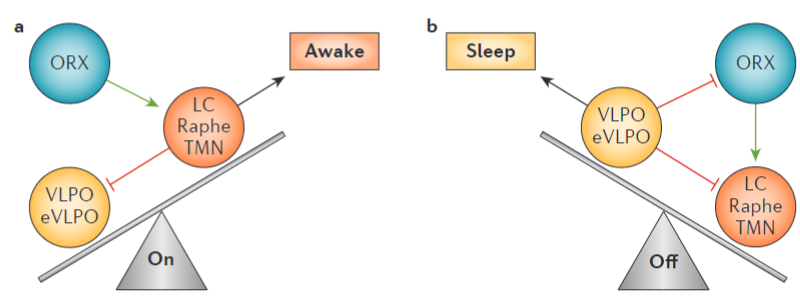
It is thus hypothesized that dysregulation of the orexin system could contribute to the abnormal hyperarousal present in insomnia, and that antagonism of orexin receptors could facilitate sleep onset and reduce night-time awakenings1,5,7.
In 2014 Suvorexant became the first dual orexin antagonist (DORA) approved by the FDA for the treatment of insomnia, and has so far earned a lot of money, around 300 million annually for Merck’s Belsomra.
In terms of efficacy, a 2021 meta-analysis7 determined that DORAs (all of the approved DORAs) were effective treatments for insomnia, with a clinically significant reduction in latency to persistent sleep (similar to sleep onset), total sleep time, and wake after sleep onset (time spent awake after initially falling asleep). These measures are particularly important because they are objective. In insomnia, patients are nearly incapable of accurately recalling how long it took them to fall asleep, how long they slept for, and how many times they woke up throughout the night, making subjective self reporting very inaccurate5,7.
In terms of safety and tolerability, around half of all patients experience at least one side effect, according to a recent meta-analysis. The most common side effects of DORAs were dry mouth, “weird dreams” (false awakenings, night terrors and the such), headaches and day-time impairment. Of note is the fact that unlike with other CNS depressants, patients were able to tell that their driving capacity was impaired. Something I found particularly strange was how “potential drug abuse” was listed, indicating a small possibility that suvorexant could be abused by certain users or that it may make users more prone to abuse other drugs7 (to be fair, people can abuse pretty much anything with the right mindset).
In conclusion: For sleep onset and wake after sleep disorders, DORAs are very good! They are far from ideal, but compared to other insomnia medications, they are significantly less addictive, cause less day-time impairment and have an OK tolerability. DORAs make for an incredibly valuable tool for the treatment of insomnia.
TAK-994 is what we call a research compound. Since some papers discussed here predate the renaming of TAK-994 into Firazorexton, the names TAK-994 and Firazorexton will be used interchangeably (by the way, the name TAK comes from Takeda, the pharmaceutical company behind firazorexton).
Firazorexton is a selective OXR2 agonist. For the treatment of narcolepsy, it acts primarily in the central nervous system by keeping monoaminergic nuclei stimulated and thereby keeping us awake (according to the flip-flop switch model of sleep). The phase 2 studies for TAK-994 were quite promising, with narcoleptic patients showing a marked improvement in Maintenance of Wakefulness Test (MWT), a test in which patients are put in a very dimly lit room, in which they have to sit on a bed and try not to fall asleep for 40 minutes. They would perform the test to determine a baseline level, then try again after 8 weeks of treatment. Also noteworthy of the study was the rate of cataplexy episodes!
The good part: Sleep outcomes
The placebo group after 8 weeks of treatment showed no improvement, going from an average of 6 minutes to fall asleep to 2.8 minutes at week 8 in the MWT.
Cataplexy events fell from 15.9 episodes per week to 10.6 episodes per week.
The Firazorexton groups showed drastic improvements for MWT in what appears to be a dose-dependant fashion, averages of +25 minutes for 30mg, +27 minutes for 90mg and +33 minutes for 180mg (oral, once daily)
Cataplexy events fell from 11-15 episodes per week to around a single (1) episode per week!
The bad part: Terrible terrible safety profiles
Around 80% of patients that received TAK-994 had adverse events with the most common adverse events being urinary urgency and frequency (You no longer pass out but are now constantly running to the bathroom). The most serious adverse event was apparent liver damage, with five patients, two from the 90mg group and three for the 180mg group, developing elevated liver enzymes, which led to the termination of all clinical trials for TAK-994. I seriously doubt that the agonism of orexin caused the liver damage, but considering the role orexins have on metabolism, it is a non-zero chance. We can only hope for the speedy development of better drugs that avoid causing liver damage, and for narcoleptics to get really good bladders. Currently, TAK-861 is being developed and until more information becomes available, this will be it for the agonist section of this article. Hopefully further research leads to a better understanding of the relationship of orexin agonists, appetite and body temperature. :)
-
References
- Muehlan C, Roch C, Vaillant C, Dingemanse J. “The orexin story and orexin receptor antagonists for the treatment of insomnia”. 2023. doi: 10.1111/jsr.13902.
- Azeez, I.A., Igado, O.O. & Olopade, J.O. “An overview of the orexinergic system in different animal species”. 2021. DOI: 10.1007/s11011-021-00761-0
- Ling Lin, Juiette Faraco, Robin Li. “The Sleep Disorder Canine Narcolepsy Is Caused by a Mutation in the Hypocretin (Orexin) Receptor 2 Gene”. 1999. DOI: 10.1016/S0092-8674(00)81965-0
- Yves Dauvilliers, Emmanuel Mignot, Rafael del Río Villegas, et al. “Oral Orexin Receptor 2 Agonist in Narcolepsy Type 1”. 2023. DOI: 10.1056/NEJMoa2301940
- Morin, C., Drake, C., Harvey, A. et al. “Insomnia disorder”. 2015. DOI: 10.1038/nrdp.2015.26
- T. E. Scammell. "Wakefulness: An eye-opening perspective on orexin neurons". 2001. DOI: 10.1016/S0960-9822(01)00466-3
- Tao Xue, Xin Wu, Shujun Chen, et al. “The efficacy and safety of dual orexin receptor antagonists in primary insomnia: A systematic review and network meta-analysis”. 2022. DOI: 10.1016/j.smrv.2021.101573